Learn How
Digatherm Can
Speed Up Your
Wellness Exams
Thermal imaging helps veterinary professionals
evaluate their patients' physiology quickly and easily
to create more efficient treatment plans sooner
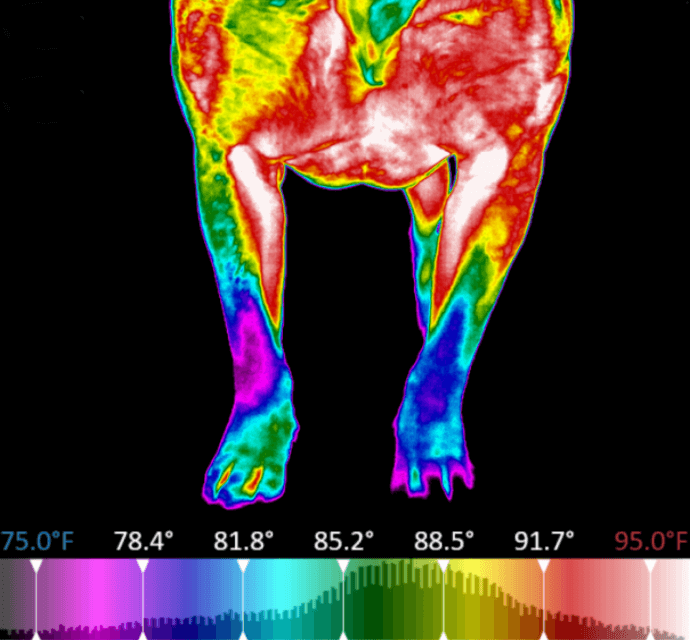


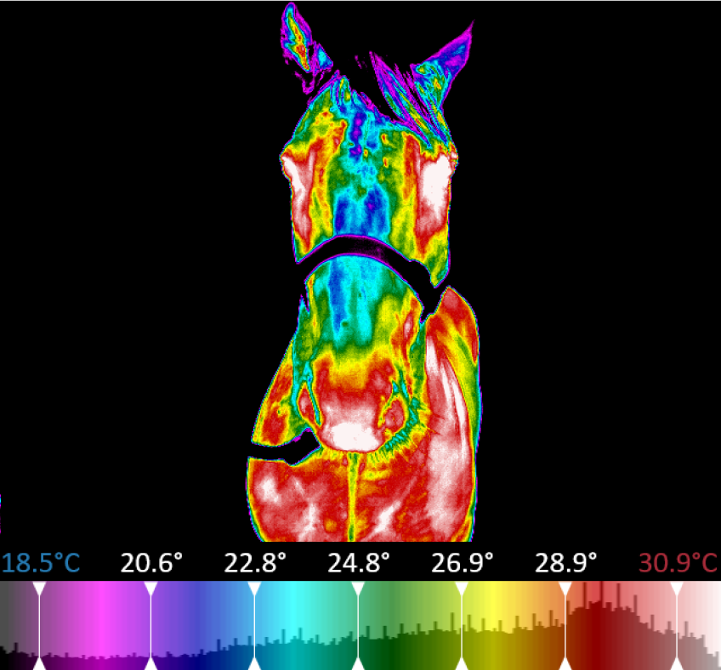
Thermal Imaging Helps Make Proactive Medicine Practical
Routinely using thermal imaging provides a simple, easy solution to practicing proactive medicine. By integrating this screening tool into your wellness, lameness, and recheck visits, you can decrease the time you spend searching for problem areas, detect issues earlier, deliver more targeted treatments, and delight clients with visual treatment progress updates.
How does thermal imaging work?
Thermal imaging uses a highly sensitive infrared camera to measure, compile, and analyze the electromagnetic energy emitted from a patient.
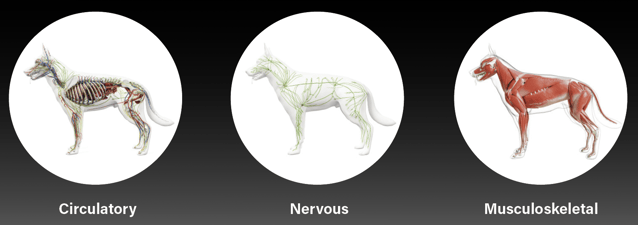
Veterinary-specific software converts the temperatures into images where these thermal emissions can be easily visualized. Temperature data directly correlates to changes within the circulatory, nervous, and musculoskeletal systems.
Digatherm® Thermal Imaging Systems
Learn more about Digatherm's veterinary tools and technology

IR Pad 640

IR Pad 320

Top Employer Guidelines for a Safer Return to Work

Temperature Screening Hospitality Guidelines for Safer Stays